
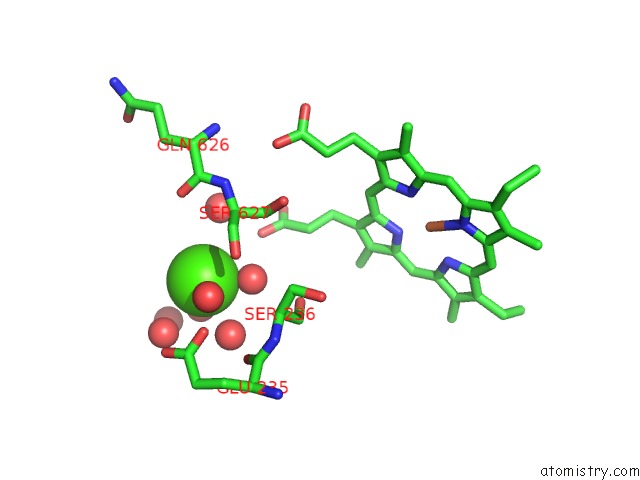
Here, we present a 3.3 Å resolution cryo-EM structure of the MCU-EMRE-MICU1-MICU2 assembly, hereafter referred to as the holocomplex, under resting conditions. Further it is not known why MICU1, in particular, is necessary among MICU1-3 for regulating the channel ( Kamer and Mootha, 2014 Patron et al., 2014 Patron et al., 2019 Payne et al., 2017 Xing et al., 2019). While structures of MICU1-3 and MCU-EMRE components have been determined ( Kamer et al., 2019 Park et al., 2020 Wang et al., 2020 Wang et al., 2014 Wang et al., 2019 Wu et al., 2019 Xing et al., 2019), it is unclear how MICU proteins interact with the channel and the mechanism by which they confer Ca 2+-dependent control to the channel is enigmatic. At the ~100 nM resting level of cytosol, MICU1-MICU2 and MICU1-MICU3 heterodimers prevent ion conduction through the channel, and they permit it when Ca 2+ levels rise ( Csordás et al., 2013 Kamer et al., 2017 Kamer and Mootha, 2014 Liu et al., 2016 Mallilankaraman et al., 2012b Paillard et al., 2017 Patron et al., 2014 Patron et al., 2019 Payne et al., 2017 Phillips et al., 2019).
IMS, which closely follows the cytosolic Ca 2+ concentration due to the permeability of the outer mitochondrial membrane, is sensed by EF-hand motifs in each protein. Studies have resolved that MICU1-MICU2 heterodimers reside in the intermembrane space (IMS) where they regulate the uniporter by responding to IMS ( Kamer and Mootha, 2014 Patron et al., 2014 Plovanich et al., 2013). Multiple models for this regulation have been proposed but issues as fundamental as the stoichiometry between the heterodimers and the channel remain unclear. The molecular mechanism by which the MICU1-MICU2 and MICU1-MICU3 heterodimers regulate the channel is a matter of intense study ( Csordás et al., 2013 Hoffman et al., 2013 Kamer et al., 2017 Kamer and Mootha, 2014 Kamer et al., 2018 Paillard et al., 2018 Patron et al., 2014 Patron et al., 2019 Payne et al., 2017 Perocchi et al., 2010 Phillips et al., 2019 Plovanich et al., 2013). The activity of the uniporter is further tuned by interactions with MCUR1 ( Chaudhuri et al., 2016 Mallilankaraman et al., 2012a Vais et al., 2015) and the MCU-like protein MCUb ( Raffaello et al., 2013). Ca 2+-dependent control is conferred by proteins that are unique to the uniporter − heterodimers of MICU1-MICU2 in most cells and of MICU1-MICU3 in neurons ( Ashrafi et al., 2019 Kamer et al., 2017 Kamer and Mootha, 2014 Patron et al., 2014 Patron et al., 2019 Payne et al., 2017 Perocchi et al., 2010 Plovanich et al., 2013).
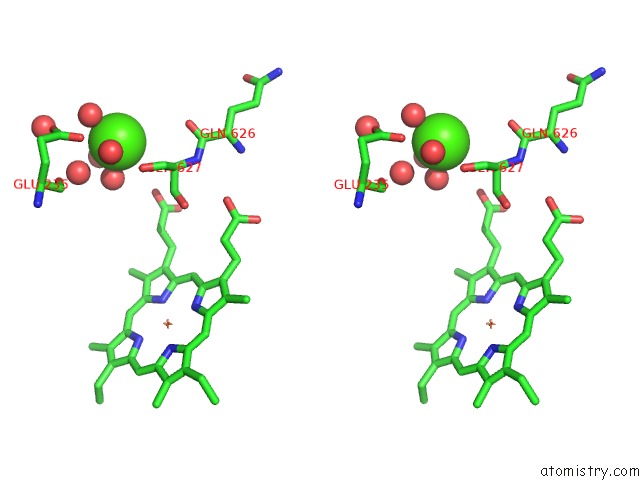
The protein MCU constitutes the pore of the uniporter through which Ca 2+ ions flow and EMRE, a small transmembrane protein that associates with MCU, is necessary for Ca 2+ uptake in metazoan organisms ( Baughman et al., 2011 Chaudhuri et al., 2013 De Stefani et al., 2011 Kovács-Bogdán et al., 2014 Sancak et al., 2013). These properties, Ca 2+ selectivity and block of ion permeation under resting conditions, are fundamentally important for preventing mitochondrial Ca 2+ overload and for maintaining the electromotive force used for ATP production because potassium and other cations outnumber Ca 2+ by more than one million to one in the cytosol. The uniporter is one of the most selective Ca 2+ channels known and it is only activated once cytosolic Ca 2+ levels reach a threshold (of approximately 0.4–3 μM) ( Csordás et al., 2013 Kirichok et al., 2004 Liu et al., 2016 Mallilankaraman et al., 2012b Payne et al., 2017). The mitochondrial calcium uniporter is a highly selective multi-subunit Ca 2+ channel of the inner mitochondrial membrane that serves as the major conduit for uptake of Ca 2+ by mitochondria and controls ATP production in response to cytosolic Ca 2+ signals ( Gunter and Pfeiffer, 1990 Kamer and Mootha, 2015 Pan et al., 2013 Pathak and Trebak, 2018).


 0 kommentar(er)
0 kommentar(er)
